7+ Freezing Point Depression Calculation
Web The freezing point depression formula uses the Clausius-Clapeyron equation and Raoults law. ΔTf change in freezing point Kf molal.

Skew T Parameters And Indices
Web By the end of this lab students should be able to.
. Molal freezing-point depression constant. Web Yet according to the freezing point depression equation the freezing point will rise during the experiment why. Web The formula for calculating the freezing point depression ΔTf using a Freezing Point Calculator is as follows.
ΔTf is the freezing point. For a dilute ideal solution the formula for freezing point. Web This freezing point depression calculator will help you determine the drop in the solutions freezing point compared to the pure solvents freezing point when you.
Web 1 Freezing-point depression is a drop in the maximum temperature at which a substance freezes caused when a smaller amount of another non- volatile substance is added. ΔTf Kf m Where. Web K f M M Freezing point depression the freezing point goes down occurs when solute is added to the pure solvent.
Know the precision of volumetric flasks and perform calculations to the correct number of. Determine the cryoscopic constant Kf for the. Some important uses of freezing point depression are listed below.
ΔTf Kf x m where ΔTf is the change in. Delta T_f the amount the freezing. Δt i K 132 C 1 512 C kg mol x 00273 kg.
Kf is the cryoscopic. Web Uses of Freezing Point Depression. Web The following formula is used to calculate a freezing point depression.
Web The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. Web Calculation of freezing point depression. In cold areas where the temperatures range from 0 o C to -15 o C.
Web The following equation is used to calculate the decrease in the freezing point. Tf Kf b i T f K f b i. The molal freezing-point depression.
Web Formula T Kf m Freezing Point Depression Coefficient Freezing Point Temperature Enter the freezing point depression constant and the molality into the. ΔTf Kf x molality Where. T f i.
T f m. The difference in temperature between the freezing point of the pure solvent and that of the solution. Web The amount of depression in the freezing point is proportional to the molal concentration of the solution and is given by the equation.
Web freezing point depression. Delta T_f k_f cdot textm cdot i labelFP Where. As ice melts it turns to liquid water and the molaity goes.
Thus the amount of depression depends on the amount of. Web The depression in the freezing point ΔTf can be calculated using the following formula. Web To calculate freezing point depression using the cryoscopic constant and the Vant Hoff factor follow these steps.
Web For example using the appropriate data in the table determine the freezing point depression of the solution that contains 241 g urea NH 2 2 CO in 485 mL of water. Use a volumetric flask to make a solution. For a dilute solution the depression in the freezing point is directly proportional to the molality of the solute.
Where Tf is the freezing point depression.
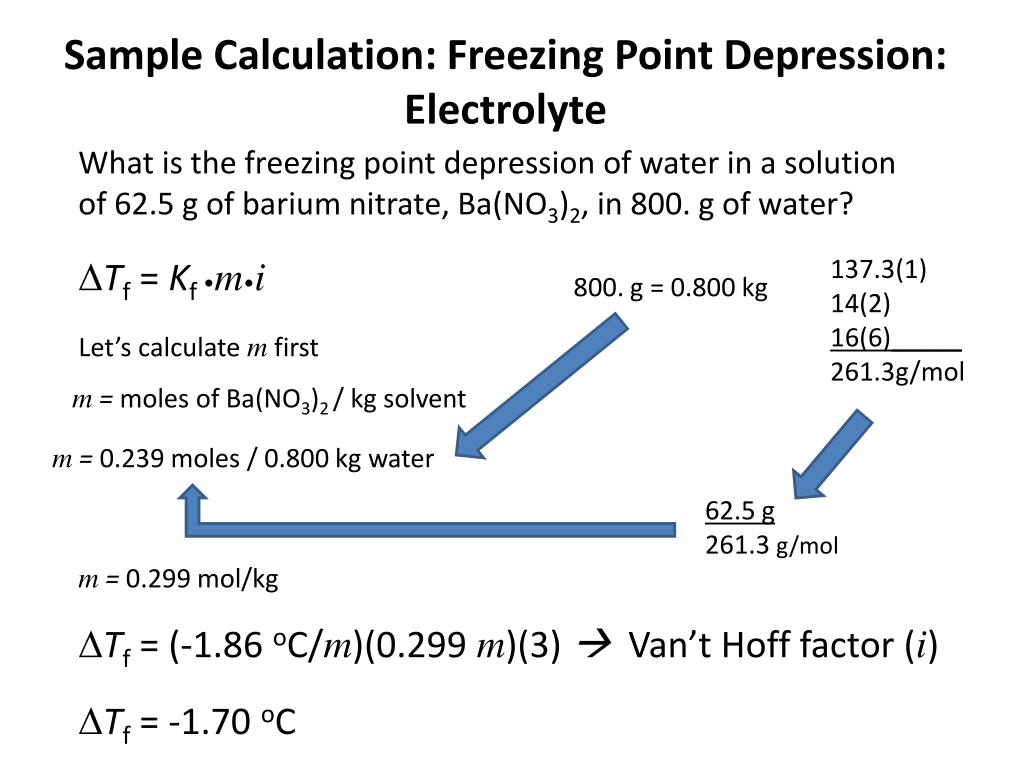
Ppt Calculations Amp Colligative Properties Powerpoint Presentation Id 5408289

Freezing Point Calculator Formula Calculator Academy

Theory A Pure Liquid Has Its Own Physical Properties Such As Boiling Point And Freezing Point But When A Solute Non Ionized Non Volatile Is Dissolved Ppt Download

Meteorological Techniques Rammb
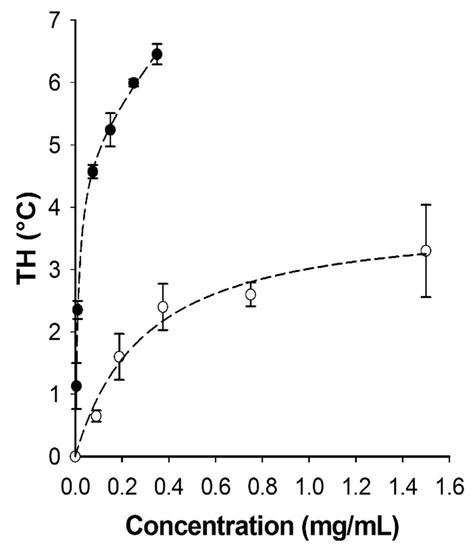
Biomolecules Free Full Text Laboratory Scale Isolation Of Insect Antifreeze Protein For Cryobiology
Chemistry 104 Molecular Weight By Freezing Point Depression
Freezing Point Depression
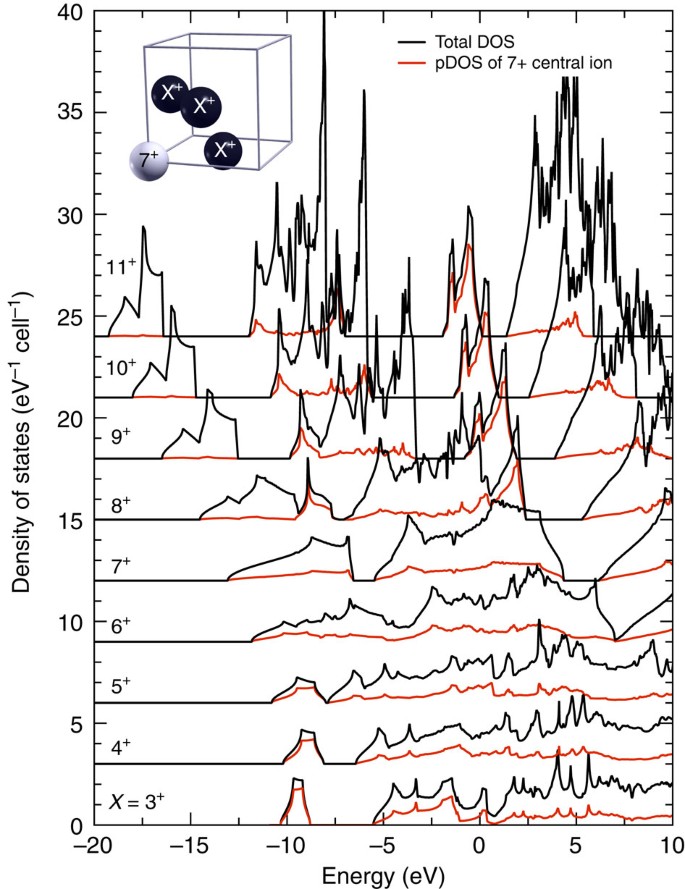
Density Functional Theory Calculations Of Continuum Lowering In Strongly Coupled Plasmas Nature Communications

Predicting Relative Freezing Point Depressions Chemistry Study Com

Freezing Point Depression Calculator

How To Calculate Freezing Point Depression The Tech Edvocate
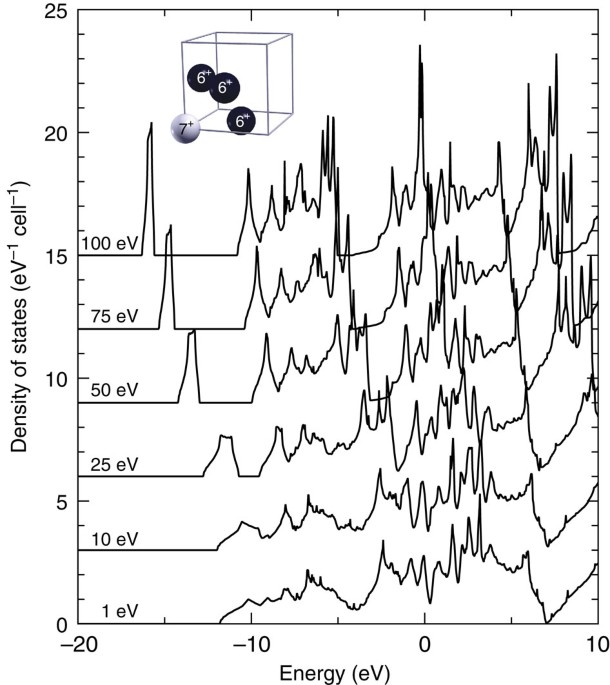
Density Functional Theory Calculations Of Continuum Lowering In Strongly Coupled Plasmas Nature Communications

Thermodynamic Equilibrium And Kinetic Fundamentals Of Oxide Dissolution In Aqueous Solution Journal Of Materials Research Cambridge Core
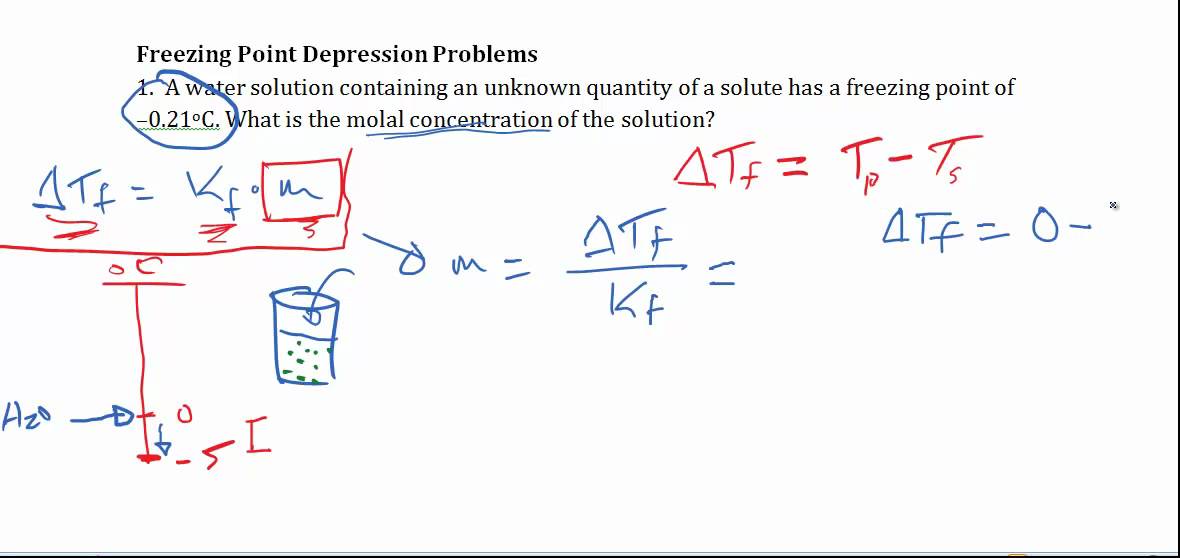
Calculating Freezing Point Depression Youtube
Dat Breakdown 06 17 2015 27aa 25ts 24pa Student Doctor Network

Freezing Point Depression Formula And Definition

Thermodynamic Equilibrium And Kinetic Fundamentals Of Oxide Dissolution In Aqueous Solution Journal Of Materials Research Cambridge Core